Regulatory Compliance
Regulatory Services
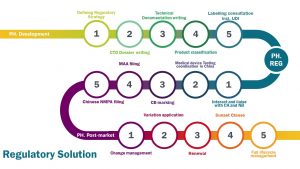
Life science industry today is a highly regulated and ever-evolving environment. The development of either a pharmaceutical product or a medical device usually takes years from initial conceptualization to commercialization and involves several phases. From the early development phase (PH.Development), going through the registration phase (PH.REG) to the post-market phase (PH.Post Market), ever increasing and complex regulatory requirements have to be met. Our expertise in dealing with these requirements can change your daily business.
Case Study 1: DHF Remediation
Our client is an established mid-sized medical device manufacturer in Western Europe and distributes medical devices internationally. Due to a lack of resources, the Design History File (DHF) of its devices was not maintained throughout the life cycle to meet the increased regulatory requirements worldwide. In preparation for the upcoming inspection, a number of DHFs of legacy medical devices needed to be reviewed for compliance to the current regulatory requirements.
We first proposed to the client to open a CAPA, which primarily served as a guide. According to the CAPA plan, we performed a gap analysis for the client. In a single document, we compiled a list of affected medical devices; the technical files guidelines, all available versions of technical files, a list of changes made to each medical devices, and the updated documents resulted from that change. This approach allowed us to determine if certain documents were missing or outdated.
From there, we created a project plan and worked with the client to assign responsibility for revising each document with the set timeline. For changes during the post-marketing phase, we re-evaluated and confirmed the change category i.e., minor or major change and the supporting documents in order to justify the change. We focused on the regulatory documents such as device description, classification and revision or creation of the device label while the client worked on technical documents for design control.
Since the devices under the remediation have been in the market for over 10 years, many DHFs were documented in paper format, which made version control of the DHFs very difficult. We agreed with the client to scan and reformat them into an electronic format for better document control.
The project took 5 months and the client eventually passed the inspection without any non-conformity finding.
Case Study 2: Repeat-use MRP
Our client is a global biotechnology leader based in Central Europe. For more than a century, the company has been involved in the research and development of cure for severe and rare diseases. The biological product we supported was registered initially in Europe through a national procedure in a single European member state and then through a Mutual Recognition Procedure (MRP) in several selected European member states. In order to further penetrate the European market, the client decided to apply for additional European licenses.
We were involved in the project as the primary regulatory responsible for the entire procedure.
Prior to the procedure, we worked on the administrative part of the submission procedure, such as compiling Module 1 of the dossier, consolidating the list of variation/renewal since the last MRP, finalizing eCTD dossier, coordinating dossier printing, labeling dossier binders, and burning the CD-ROM according to individual national requirements. In addition, we dispatched the complete dossier to the client’s subsidiaries and further to the local national authorities and negotiated a time slot with the Reference Member State (RMS).
During the MRP procedure, there was frequent communications via email and telephone conference with all parties involved, i.e., the applicant, the client’s European subsidiaries and the national authorities (16 in total). This was to ensure that all additional documents required to address questions raised during the procedure was available. We adhered closely to the schedule of the procedure, effectively answered all questions raised on Day 60 and Day 75. The entire procedure was carried out smoothly and successfully through Day 90.
Within the one-year project timeframe, we assisted the client in expanding product licensing to additional 15 European countries.