European regulations (EU MDR and EU IVDR) mandate that medical device manufacturers, distributors and importers effectively handle complaints, report serious adverse events (SAEs) and monitor trends in non-serious events. But what are complaints, adverse events (AEs) and SAEs? How do they differentiate from each other and how do they all fit together from a quality management perspective (Figure 1)?
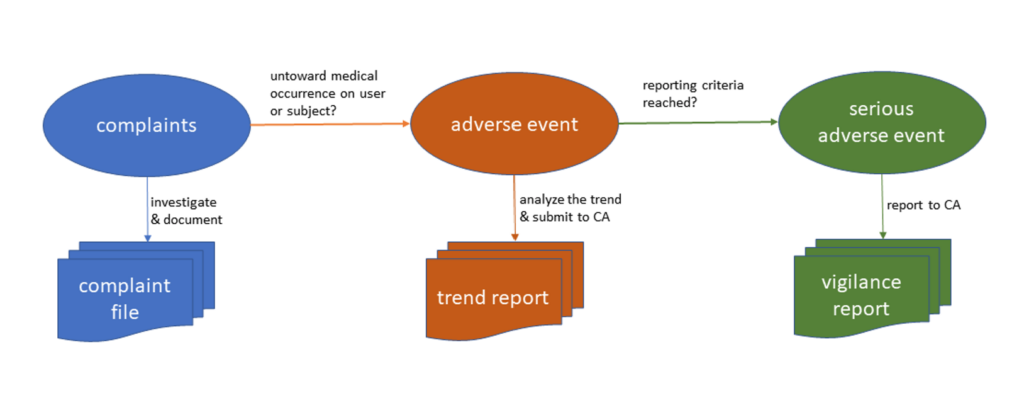
1. Relationship of complaint, AE and SAE
Distributor and importer must document complaints and forward them to manufacturers for evaluation. Manufacturers then review and assess these complaints to determine whether an investigation is necessary. If no investigation is deemed necessary, the rationale must be documented.
Although the EU MDR and EU IVDR require economic operators to manage complaints, they don’t define what constitutes a complaint, whereas ISO 13485 provides this definition. In contrast, the EU MDR & IVDR clearly defines AE and SAE.
Complaint acc. to ISO 13485
written, electronic or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, usability, safety or performance of a medical device that has been released from the organization’s control or related to a service that affects the performance of such medical devices.
Adverse event acc. to EU MDR & (IVDR)
means any untoward medical occurrence, (inappropriate patient management decision,) unintended disease or injury or any untoward clinical signs, including an abnormal laboratory finding, in subjects, users or other persons, in the context of a clinical investigation (a performance study) whether or not related to the investigational device (performance study).
Serious adverse event acc. to EU MDR & (IVDR)
means any adverse event that led to any of the following:
(a) (a patient management decision resulting in death or an imminent life-threatening situation for the individual being tested, or in the death of the individual’s offspring.)
(b) death,
(c) serious deterioration in the health of (the individual being tested or the recipient of tested donations or materials,) that resulted in any of the following:
- life-threatening illness or injury,
- permanent impairment of a body structure or a body function,
- hospitalisation or prolongation of patient hospitalisation,
- medical or surgical intervention to prevent life-threatening illness or injury or permanent impairment to a body structure or a body function,
- chronic disease,
(d) foetal distress, foetal death or a congenital physical or mental impairment or birth defect,
Based on these definitions, a complaint refers to any deficiencies related to medical devices. An adverse event may initially be reported as a complaint and only becomes an AE when a subject or user experiences an unintended medical occurrence with clinical signs.
An AE meeting reporting criteria must be reported to the competent authority (CA). If the AE doesn’t result in any serious outcome for users or subjects, reporting to the CA isn’t required. However, manufacturers must report any statistically significant increase in the frequency or severity of AEs that are not serious or are expected undesirable side-effects that could significantly impact the benefit-risk analysis (Art. 88, EU MDR & Art. 83 EU IVDR).
Trend reporting can be challenging for many manufacturers as it involves establishing an appropriate reporting threshold and identifying significant trend changes during periodic data reviews. This difficulty often arises from the absence of a systematic trending strategy, such as the lack of categorization for adverse events.
2. Adverse event code
In 2020, IMDRF released the first edition of guidance on adverse event code (IMDRF/AE WG/N43FINAL:2020 (Edition4). These codes are primarily based on the US FDA’s issue codes. Since its release, this document has been well-referred by medical device industry and regulators in Europe. The adverse event codes have even been integrated into the European vigilance reporting template (MIR). This development requires manufacturers to thoroughly understand adverse event codes to select the appropriate ones for reporting purposes.
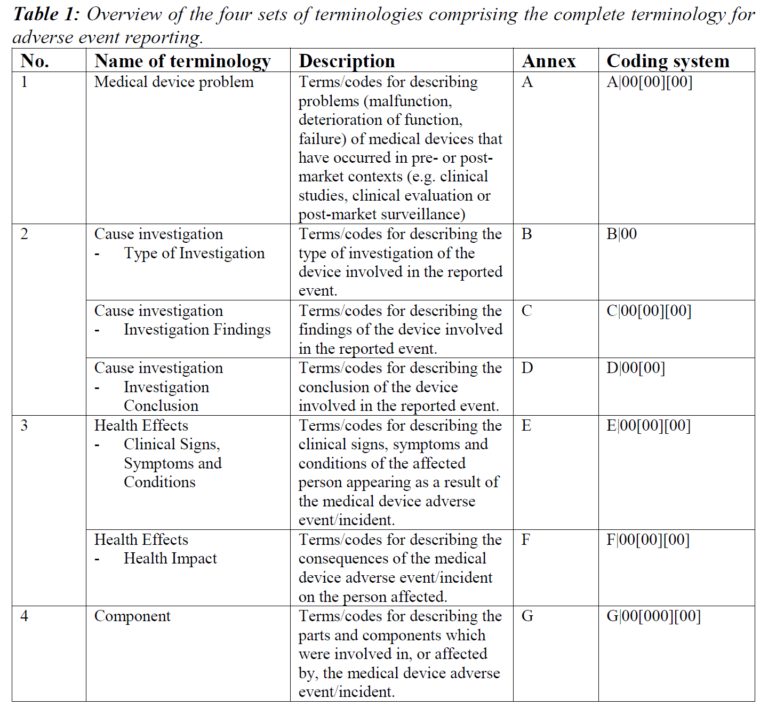
The complete adverse event terminology defined in the IMDRF guidance comprises four distinct sets of terminologies, associated with seven annexes (A to D), as outlined in IMDRF, Table 1:
- Annex A: medical device problem terminology,
- Annex B to D: cause investigation terminology,
- Annex E to F: health effects terminology,
- Annex G: components terminology.
The code structure for the nomenclature is expressed as X[nn][nn][nn], where X represents the corresponding annex and [nn] represents the level within each annex as per the IMDRF guidance.
Coding adverse events reduces ambiguity and is more user-friendly compared to narrative text for signal detection i.e., identifying potential novel risks and analyzing trends in non-SAEs, thereby enabling faster response by regulators and manufacturers.
3. Reporting vigilance cases and trend reporting
In practice, if a customer complains that a defibrillator failed to deliver a shock, this complaint can quickly be categorized as an adverse event according to its definition. This is because the failure to administer a shock to a patient with ventricular arrhythmia involving both faulty device and patients with clinical signs. To utilize the adverse event coding system, the manufacturer must determine the appropriate A-, B-, C-, D-, E-, F- and/or G-code sequentially at each step of its investigation. Below is a practical example of all adverse event coded identified by consulting the IMDRF guidance annexes.
- A071301 describes the medical device problem associated with the device’s failure to deliver electrical energy.
- B01 describes the type of investigation– testing of actual device was conducted to establish its functional and other properties.
- C0404 indicates that the investigational findings showed the adverse event was due to unintended compatibility.
- D09 is the investigational conclusion indicating that the problem was caused by inadequate information in the instruction for use.
- E060110 describes the clinical signs of the patient involved, which was ventricular arrhythmia.
- F05 describes the resulting consequences of the medical device adverse event on the patient, which was the delayed treatment.
4. Global impact
Today, regulators are increasingly communicating and sharing post-market surveillance information at the international level. Using common terms and definitions facilitates analysis of safety, quality and performance information, which in turn makes global sharing easier. Common terminology also enhances the accuracy and reliability of information exchanged about the device adverse events.
Coding AEs according to the IMDRF guidance ensures consistent reporting across multiple jurisdictions and reduces the complexity of managing multiple coding system when preparing adverse event reports for various regions.
