Buying and selling is an important part of doing business today, made even more important by globalization. At the heart of a buying or selling decision is a right balance of price and quality. But how can we find a right balance between price and quality when buying and selling? For most commercial commodities, this process is relatively straightforward by finding the source for one’s need, taking into account cultural and regional market diversities.
However, for healthcare products such as medical devices intended to diagnose, prevent, prognose or alleviate disease or injury, their safe use and sound clinical benefit must be ensured, which is why the medical devices industry is highly regulated. Not only manufacturers who must demonstrate the compliance of medical device before placing them on the market, but also other parties involved in the supply chain, e.g., importers and distributors up to the end users have their own regulatory requirements to comply with, e.g., verification of product approval or certification and agreement on supply quality and etc., before a final deal is struck.
To connect these dots and facilitate the necessary communication across a board of parties involved in the medical device supply chain, we have been engaged ourselves on development of an online tool. The tool is in the final stage of the development and is about to be launched.
In addition to our direct forthcoming online services, our professional team provides tailored support in finding a suitable manufacturer such as OEM & ODM & OBM, distributor or service provider meeting your needs, following the six steps of sourcing workflow. We act as a liaison between you and the supplier to facilitate the entire process.
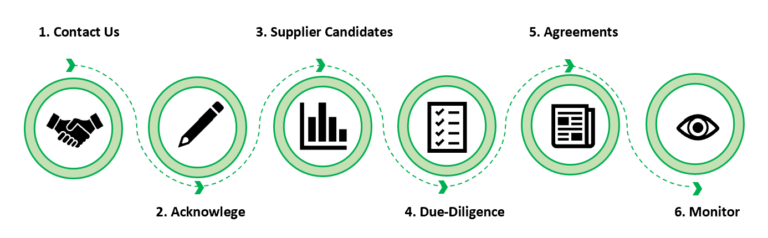
Contact us by filling up the Contact Sourcing Form and providing your project plan, stakeholder/product requirements;
We confirm your request within 24 hours;
Provide you with a list of potential supplier candidates that have the business and operational capability for an initial assessment;
Based on your selection, we conduct a due-diligent review to assess the technological, business and quality risks of the particular supplier and its ability to fulfill the specific requirements. Document evaluation and selection criteria, decision and rationale, and all correspondence and hand it over to you;
On your behalf, we draft, negotiate and conclude a supply/distribution agreement and a supply quality agreement, taking into account the regulatory and quality requirements.
- Monitor the performance of the supplier to ensure that it still meet the original evaluation and selection criteria or any new/revised criteria.