The US-FDA has issued a draft guidance to address key aspects of drug delivery performance for devices, and combination products that include device constituent parts designed to deliver a human drug and biological products. These key aspects, referred as essential drug delivery outputs (EDDO), are critical to ensure the reliable and accurate functioning of the drug delivery system.
This guidance applies to a variety of drug delivery devices, such as syringes, injectors, infusion pumps, nasal sprays, inhalers, nebulizers, and vaginal systems. By focusing on EDDOs, the guidance emphasizes design outputs critical to delivering the intended drug dose to the intended site.
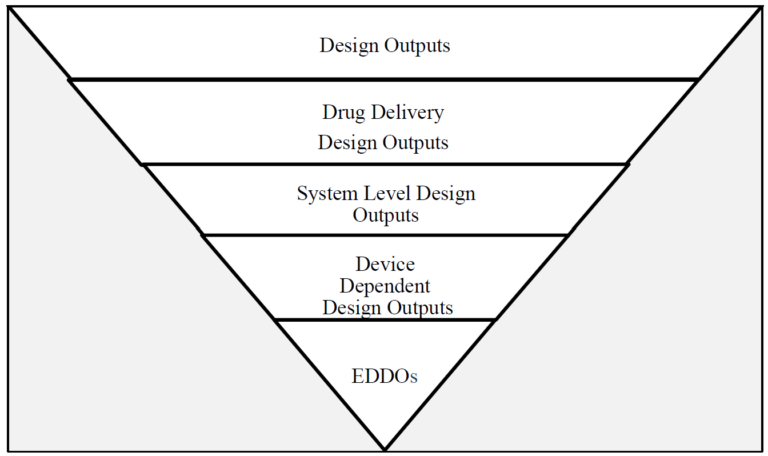
Design output and EDDOs
Design output is defined as the results of a design effort at each design phase and at the end of the total design effort (21 CFR 820.3(g)). The finished design output serves as the basis for the device master record. The total finished design output consists of the device, its packaging and labeling, and the device master record.
EDDOs are a specific subset of design outputs that directly dependent on the device design and essential for the drug delivery function. These outputs ensure proper device functioning to enable accurate and reliable drug delivery. While drug delivery as a whole involves preparation and administration (including initiation, progression and completion), EDDOs focus specifically on system level outputs independent of user factors.
Steps to identify, control and maintain EDDOs
The guidance explains three steps towards identify, control and maintain the EDDOs out of design outputs.
1. Identification:
This draft guidance introduces a filtering process to help manufacturers identify EDDOs from existing design outputs (see flowchart). This process begins by considering all design outputs, then narrowing the focus to system level outputs critical for the device’s performance. It distinguishes between:
- user-dependent design outputs, which relate to usability and are addressed in separate FDA guidance.
- device-dependent design outputs, which form the EDDOs directly tied to the device’s design and functionality.
By excluding usability-related outputs, the identification process ensures that EDDOs are limited to aspects that are device design-dependent and necessary for consistent drug delivery.
2. Verification and validation:
Verification and validation ensure that the device meets the quality standard for drug-delivery function. The final finished must consistently conform to design outputs across all manufactured lots. A robust control strategy is essential especially for high risk devices and should include:
- upstream controls, such as in-process controls, control of process parameters, control of incoming materials, and purchasing controls.
- downstream controls, such as lot release testing.
The control strategy developed should be risk-based to ensure effective oversight of the manufacturing process and the drug delivery function.
3. Maintenance:
The guidance also address the need to maintain the integrity of EDDOs throughout the product lifecycle. Any changes made during clinical development or post-market that could adversely impact the EDDOs should be evaluated to ensure the quality and reliability of drug delivery function remain intact.
Practical applications and regulatory submissions
The draft guidance provides practical examples for common drug delivery devices such as prefilled syringes and autoinjectors. These examples illustrate how manufacturers can systematically identify EDDOs in compliance with the guidance, offering a step-by-step approach from the FDA’s perspective.
Finally, the guidance outlines the FDA’s current expectations for data regarding EDDOs to be submitted during Investigational New Drug (IND), Investigational Device Exemption (IDE), and marketing applications. By aligning with this guidance, manufacturers can ensure their submissions address the agency’s standards for drug delivery performance.