Safety and performance are absolute regulatory requirements that the manufacturer must demonstrate before placing their device on the market. The EU MDR Annex I defines the general safety and performance requirements (GSPRs) for conformity assessment of the medical devices.
Out of approx. 20 pages of GSPRs, two requirements (Clause 1 and 5, see below) have become even more important in recent years in the global regulatory landscape. The reason behind this tighten regulatory control is twofold. Firstly, medical practice increasingly relies on medical devices for diagnosis and treatment of patients. As healthcare evolves, less skilled users including patients themselves are now using medical devices, and medical devices are becoming more complex. This has led to an increased use errors caused by inadequate medical device usability. The manufacturers are therefore required to create sufficient usability data to demonstrate that users can operate and interact with the devices safely through validation testing such as usability tests.
(b) give consideration to the technical knowledge, experience, education, training and use environment, where applicable, and the medical and physical conditions of intended users (design for lay, professional, disabled or other users).
Secondly, before the entry of the EU MDR, clinical requirements were not universally implemented throughout the EU, despite many being presented in the form of guidance and standard documents. With the implementation of the EU MDR, the manufacturers are now required to collect sufficient clinical data to demonstrate a device’s ability to achieve its intended purpose and provide a clinical benefit. This has made clinical investigation a crucial aspect of the conformity assessment process.
Usability study and clinical investigation are typically not conducted at the manufacturers’ premises. Here are the differences between the two and how they complement each other:
1. Usability studies in brief
Usability is created to make the user interface easier for the user to perceive information presented by the user interface, to understand and to make decisions based on that information, and to interact with the medical device to achieve specified goals in the intended use environments. Many of these factors can influence safety and performance to various extents.
Usability is characteristic of the user interface that facilitates use and thereby establishes effectiveness, efficiency and user satisfaction in the intended use environment (IEC 62366-1: 2015).
User interface is the means by which the user and the medical device interact which includes all the elements of the medical device with which the user interacts including the physical aspects of the medical device as well as visual, auditory, tactile displays and is not limited to a software interface (IEC62366-1: 2015).
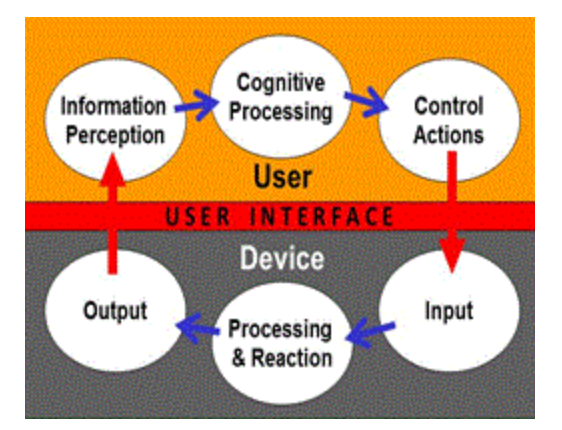
a) Purpose: A usability study assesses how users interact with a device, focusing on ease of use, user interface, instructions for use, and potential use errors. As a part of usability engineering, usability tests aim to demonstrate that the intended users can use the devices without serious use errors or problems, for the intended uses and under the expected use conditions.
b) Method: Usability studies are often conducted through simulations or mock-ups to observe how users handle the device in a controlled environment, e.g. in a conference room with the devices and the users.
c) Data Collected: Data collected from a usability study includes user feedback, error rates, time to complete tasks, and overall user satisfaction.
d) Focus: Usability studies primarily focus on the device’s design and user interface, aiming to optimize user experience and reduce the risk of use errors.
2. Clinical investigations in brief
Clinical investigation means any systematic investigation involving one or more human subjects, undertaken to assess the safety or performance of a device.
a) Purpose: A clinical investigation evaluates the safety and performance of a medical device in real-world clinical settings with actual patients.
- to establish and verify that, under normal conditions of use, a device is designed, manufactured and packaged in such a way that it is suitable for one or more of the specific purposes of medical device, and achieves the performance intended as specified by its manufacturer;
- to establish and verify the clinical benefits of a device as specified by its manufacturer;
- to establish and verify the clinical safety of the device and to determine any undesirable side-effects, under normal conditions of use of the device, and assess whether they constitute acceptable risks when weighed against the benefits to be achieved by the device (EU MDR Art. 62).
b) Method: Clinical investigations involve testing the device on patients under controlled and monitored conditions. Unlike usability tests which are conducted in a simulated environment, clinical investigation involves human subjects and therefore, shall be authorised by both ethic committee and competent authority first before human subjects recruitment.
c) Data Collected: Data collected from clinical investigations includes patient outcomes, adverse events, device performance, and overall effectiveness.
d) Focus: Clinical investigations focus on assessing how well the device achieves its intended clinical purpose, evaluating its safety, efficacy, and potential benefits compared to existing treatments.
3. The relationship between usability tests and clinical investigations
While both usability studies and clinical investigations are essential, they serve different purposes and complement each other in the regulatory process.
Usability studies help improve the device’s design and user interface, reducing the risk of use errors. This data can inform the design of the device before clinical investigation.
Clinical investigations provide crucial real-world data on the device’s safety and effectiveness in patient populations. This data validates the device’s performance in actual clinical use.
As mentioned, usability tests are typically conducted in a simulated-use environment i.e., without human subjects involved, but when necessary, usability data can also be collected under conditions of actual use or as part of a clinical investigation if simulated-use test methods are inadequate to evaluate users’ interactions with the device. For example, testing a prosthetic limb or a hearing aid programming device under simulated use conditions might not capture all real-world user experience. Likewise, results from testing a home dialysis machine in a conference room might not fully represent its use in a residential environment. However, it is important to conduct usability tests in a simulated-use conditions before involving human subjects to ensure that the device is well designed and safe in actual clinical investigation (FDA guidance).
When determining whether a usability test also qualifies as a clinical investigation under the EU MDR, factors to be considered include the scope and purpose of testing, as well as how human subjects are exposed to the device. If a usability test involves exposing users to risk related to the device or if poor usability may lead to patient or user safety risks, it may be classified as a clinical investigation (MDCG 2021-6 rev. 1).